Unlocking the potential of lithium-sulfur batteries
by Amber Rose for Argonne Information
Lemont IL (SPX) Jan 14, 2025
Lithium-ion (Li-ion) batteries are an integral a part of society, from cellphones and laptops to electrical automobiles. Whereas Li-ion batteries have been a serious success thus far, scientists worldwide are racing to design even higher ?”past Li-ion” batteries within the shift towards a extra electrified world. Industrial Li-ion batteries are much less energy-dense than different batteries and depend on comparatively costly substances, corresponding to cobalt and nickel compounds, that are additionally closely depending on susceptible provide chains.
One of many extra promising options to Li-ion batteries are lithium-sulfur (Li-S) batteries, which have an anode of lithium metallic and a cathode of sulfur. This electrode pairing guarantees two to 3 occasions greater vitality densities and diminished prices, whereas additionally utilizing Earth-abundant sources.
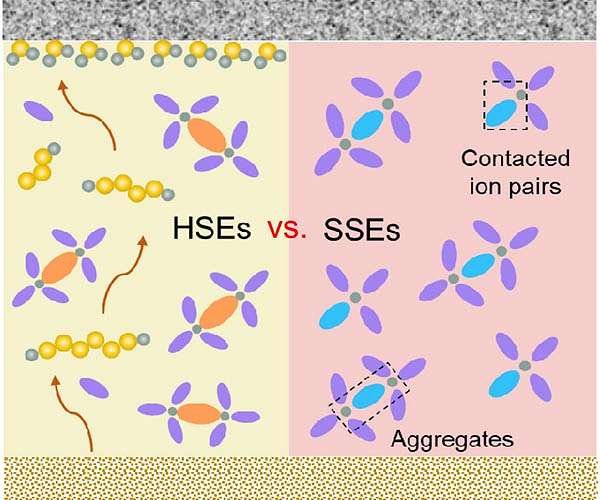
However these batteries don’t come with out their very own challenges, together with a brief cycle life because of the undesirable migration of polysulfide ions and the uneven distribution and incidence of chemical reactions throughout the system.
By growing an revolutionary additive for the electrolyte, researchers on the U.S. Division of Vitality’s (DOE) Argonne Nationwide Laboratory are making progress towards addressing these issues which might be limiting the widespread adoption of Li-S batteries.
In Li-ion batteries, lithium ions are saved within the areas between layers of the cathode materials and transfer backwards and forwards between the cathode and anode throughout charging and discharging.
Li-S batteries, nevertheless, depend on a distinct course of. In these cells, lithium ions transfer between the cathode and anode by a chemical response. Elemental sulfur from the cathode is transformed into polysulfide compounds – composed of sulfur atom chains – a few of which might dissolve within the electrolyte. Due to this solubility, a ?”shuttling” impact happens, the place the polysulfides journey backwards and forwards between the cathode and the anode. This shuttling leads to lack of materials from the sulfur cathode as a result of it’s deposited on the anode, which limits the general battery cycle life and efficiency.
Quite a few methods have been proposed to mitigate polysulfide shuttling and different challenges. One such technique, utilizing an additive within the electrolyte, has lengthy been regarded as incompatible resulting from chemical reactivity with the sulfur cathode and different battery elements. Argonne chemist Guiliang Xu and his group have created a brand new class of additive and located that such components can truly enhance battery efficiency. By controlling the way in which the additive reacts with sulfur compounds, researchers are higher in a position to create an interface between the cathode and electrolyte that’s essential to facilitate simple transport of lithium ions.
“The additive, referred to as a Lewis acid additive, is a salt that reacts with the polysulfide compounds, forming a movie over the complete electrode,” Xu mentioned. ?”The hot button is to have a minor response to kind the movie, with no steady response that consumes the fabric and reduces vitality density.”
The additive types a movie on each the anode and the cathode, suppressing the shuttle impact, bettering the steadiness of the cell and selling an ion transport ?”freeway” all through the electrode. This electrolyte design additionally minimizes sulfur dissolution and enhances response homogeneity, enabling using components that had been beforehand thought-about incompatible.
To validate the idea, the researchers in contrast their electrolyte with the additive to a standard electrolyte utilized in Li-S batteries. They noticed a major discount in polysulfide formation. The brand new electrolyte confirmed very low dissolution of polysulfides, which was confirmed with X-ray methods. Additional, they tracked the response habits throughout battery charging and discharging. These experiments made use of Argonne’s Superior Photon Supply (APS) and Brookhaven Nationwide Laboratory’s Nationwide Synchrotron Mild Supply II, each DOE Workplace of Science consumer services, which confirmed that the electrolyte design minimized the dissolution and formation of polysulfides.
“Synchrotron methods present highly effective instruments for characterizing battery supplies,” mentioned Tianyi Li, a beamline scientist on the APS. ?”Through the use of X-ray diffraction, X-ray absorption spectroscopy and X-ray fluorescence microscopy on the APS, it was confirmed that the brand new interface design successfully mitigates well-known points together with polysulfide shuttle. Extra importantly, this interface enhances ion switch, which helps to scale back response heterogeneities.”
Xu added, ?”With additional optimization and improvement of sulfur electrodes, we imagine Li-S batteries can obtain greater vitality density and higher total efficiency, contributing to their industrial adoption.”
One other main problem for Li-S batteries is the steadiness of the lithium metallic – it reacts simply and poses security considerations. Xu and his group are engaged on growing higher electrolytes to stabilize the lithium metallic and cut back the flammability of the electrolyte, guaranteeing the protection of Li-S batteries.
On the APS, Beamline 20-BM was used for X-ray absorption spectroscopy to probe the solubility of polysulfide. Beamline 17-BM was used for X-ray diffraction imaging to discover the homogeneity or heterogeneity of the complete cell. Beamline 2-ID was used for X-ray fluorescence mapping to verify solubility of the electrode materials and to watch the migration of sulfur in standard electrolytes.
Different contributors to this work embrace Chen Zhao, Heonjae Jeong, Inhui Hwang, Yang Wang, Jianming Bai, Luxi Li, Shiyuan Zhou, Chi Cheung Su, Wenqian Xu, Zhenzhen Yang, Manar Almazrouei, Cheng-Jun Solar, Lei Cheng and Khalil Amine.
The outcomes of this analysis had been printed in Joule. The examine was funded by the Car Applied sciences Workplace of DOE’s Workplace of Vitality Effectivity and Renewable Vitality.
Analysis Report:Polysulfide-incompatible additive suppresses spatial reaction heterogeneity of Li-S batteries
Associated Hyperlinks
Argonne National Laboratory
Powering The World in the 21st Century at Energy-Daily.com
Trending Merchandise











